DexCom, Inc. is a company that develops, manufactures, produces, and distributes continuous glucose monitoring (CGM) systems for diabetes management. It operates internationally with headquarters in San Diego, California, and has manufacturing facilities in Mesa, Arizona and Batu Kawan, Malaysia.
Dexcom was founded in 1999 by Scott Glenn, John Burd, Lauren Otsuki, Ellen Preston and Bret Megargel. In 2006, Dexcom received U.S. Food and Drug Administration (FDA) approval and launched the Dexcom STS Continuous Glucose Monitoring System, which is a three-day sensor that provides up to 288 glucose measurements for every 24 hours. Dexcom received approval of the second generation product, the Seven Continuous Glucose Monitoring System, in May 2007. This device improved on accuracy and extended use from three to seven days.
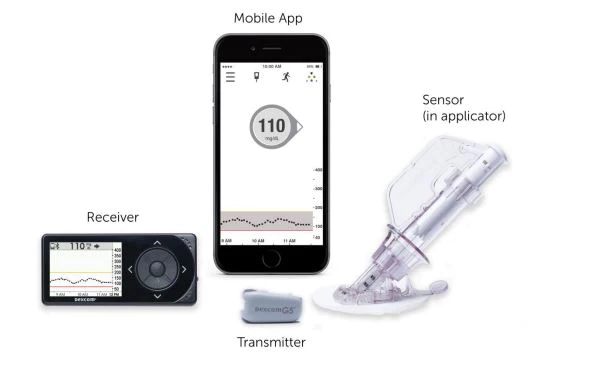
In 2016, Dexcom G5 Mobile is the world’s first real time continuous glucose monitoring (CGM) system approved for adults and children 2 years and older. The Dexcom G5 Mobile CGM System features the accurate glucose sensor on the market while providing enhanced mobility and flexibility to view and share personal glucose data and trends.
The only CGM with single digit MARD (Mean Absolute Relative Difference), the Dexcom G5 Mobile comes with the longest-wear sensor on the market and features customizable alerts and a built-in low glucose alarm (55 mg/dL alarm) to warn patients of highs and lows and how quickly they may be happening through a simplified mobile interface. Data from the Dexcom G5 Mobile can be integrated with Dexcom CLARITY™, a Cloud-based reporting software, for personalized, easy-to-understand analysis of trends that may improve diabetes management.
The FDA approved the Dexcom G7 in December 2022 for use as a standalone device, making it the most precise CGM that is currently available in the United States.
According to en.wikipedia.org; prnewswire.com. Source of photos: internet








